“Spotlight Series” is a group of posts designed to introduce you to the other members of the science party on the research expedition.
Keiran Swart

Keiran Swart on the deck of the Endeavor. He is cleaning his net following one of the tows (how he collects organisms).
Keiran Swart was born and raised in Miami.
Educational background: My undergraduate degree is from the University of Miami, where I studied geosciences, and my program had an emphasis on carbonate geology and sedimentology (for example, reef systems). I am currently a graduate student in geosciences at Princeton.
How did you become interested in science? A youth of Carl Sagan. Actually, my parents are both geologists – my dad is also a geochemist, like me, my mom is more of a classical geologist, core-work and the like. I grew up in it and always assumed I’d be a scientist.
Research Interests:
My main research interest is foraminifera – small (200µm) Protista that exist everywhere in the ocean. They are like little microcosms of coral reefs because they host symbiotic life. They are also calcareous – they contain calcium carbonate in their shells and have been a focus of geochemical study for over fifty years. The oxygen isotopic ratio in their shells has been used to make judgments about atmospheric conditions, from temperature changes and other properties, a relationship discovered by Cesare Emiliani (incidentally, he’s also from Miami).
Foraminifera serve as geochemical proxies because they are representative across the ocean, are abundant, and are continuous (meaning they are everywhere and always present). We can’t directly measure what the Earth was like a long time ago, but we can look at foraminifera to give us an idea. My work is looking at the reconstruction of the past environment with foraminifera.
With foraminifera, there’s almost an endless amount of stuff you can do with it. For example, inside a fossil, all that’s left is the shell, but the shell isn’t just calcium carbonate – it’s built on a web of protein that is an almost inconsequential component of the total mass (less than a percent). Looking at this tiny organic component can help determine many different things such as degree of nutrient consumption or water mass movement. My group tries to unify different parts of the field, including modeling and studying modern analogs, to look at how the foraminifera population tracks environmental changes on a glacial interglacial timescales.
Research aboard the R/V Endeavor:
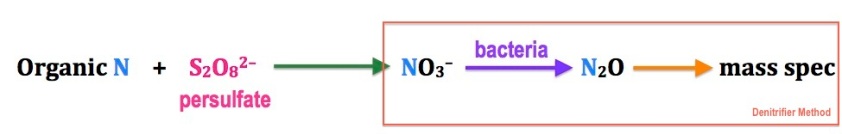
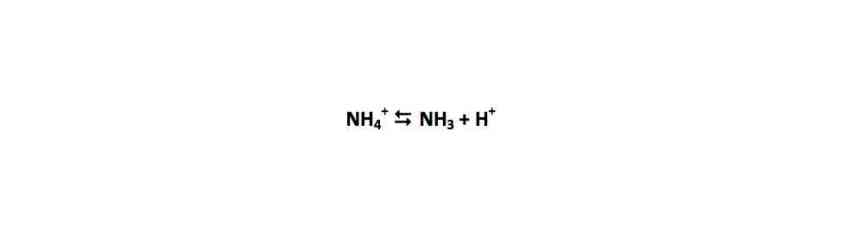
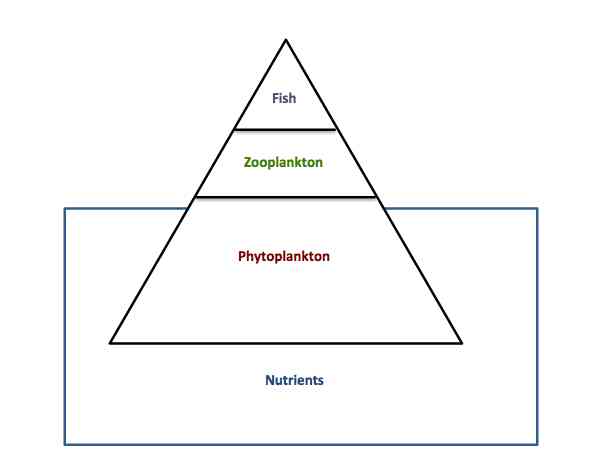
I want to look at the carbon isotopic composition of foraminifera, which is partly based on the trophic enrichment of isotopes (essentially, you are what you eat), Original photoautotrophic bacteria create new organic matter (carbon) that represents the conditions the bacteria come from and the nutrients available – for nitrogen, this means nitrate or ammonium, and for carbon, carbon dioxide or bicarbonate. The photoautotrophs are the bottom of the food web. They are eaten by something else, which undergoes metabolism and excretes waste (this goes on all the way up the food web). As we go up the food web though, the lighter isotopes (14N and 12C) tend to be excreted more than the heavier ones (15N and 13C).
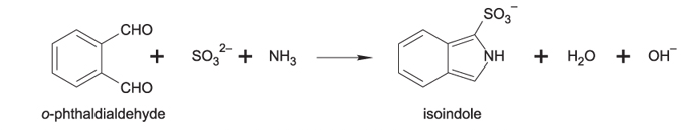
I’m interested in what controls this variability. To analyze, I pick out the species of interest, then incinerate them to produce carbon dioxide and nitrogen gases. The gases are then put through a GC (gas chromatograph – this separates the gases) and a mass spec to look at the mass profiles. From these, I can look at both the carbon and nitrogen isotope ratios and correlate with different environmental permutations.
I am interested in the isotopic composition of foraminifera. Because they are heterotrophs and eat everything, and have a symbiotic relationship with organisms that fix new carbon and trap it, there exist a complicated mesh of processes that affect the signal. Therefore, I need to get a good grasp on how the carbon isotopic composition varies across the food web and from different environmental conditions (including temperature, CO2 content in water, and pH, among others).
Basically, I collect water samples for alkalinity and carbon testing, and filter for organic matter. At the process stations, I do plankton and zooplankton tows to collect those organisms.
The phytoplankton and zooplankton get crushed up and combusted in silver foil, and then undergo a series of redox reactions to trap gases. This allows me to get the isotopic composition of the gas and see how it varies. In the future, I hope to incorporate species levels and distinctions as well.
With my water samples, I do titration alkalinity (add acid until there is a rapid pH change) and measure the change in carbon. Carbon is very complex but, essentially, CO2 is in equilibrium with H2O in various forms: carbonic acid (H2CO3), bicarbonate (HCO3—), or, carbonate (CO32–), and is constantly re-equilibrating. To look at what is biologically accessible to the foraminifera, I record a bunch of different conditions, like those I mentioned earlier – temperature, pH, etc.
Plans for the future: I want to stay in academia, though I have given thought to doing work for a nuclear regulatory agency (enforcing test ban treaties works by measuring the decay of radioactive isotopes) or work for the and environmental NGO or Greenpeace, but I do think I want to end up in academic doing research.
Back to Ms. B:
Mr. Swart is extremely passionate about his research and I hope that came across in his responses – having a sincere passion for what you are studying is essential!
Until next time,
Ms. B.